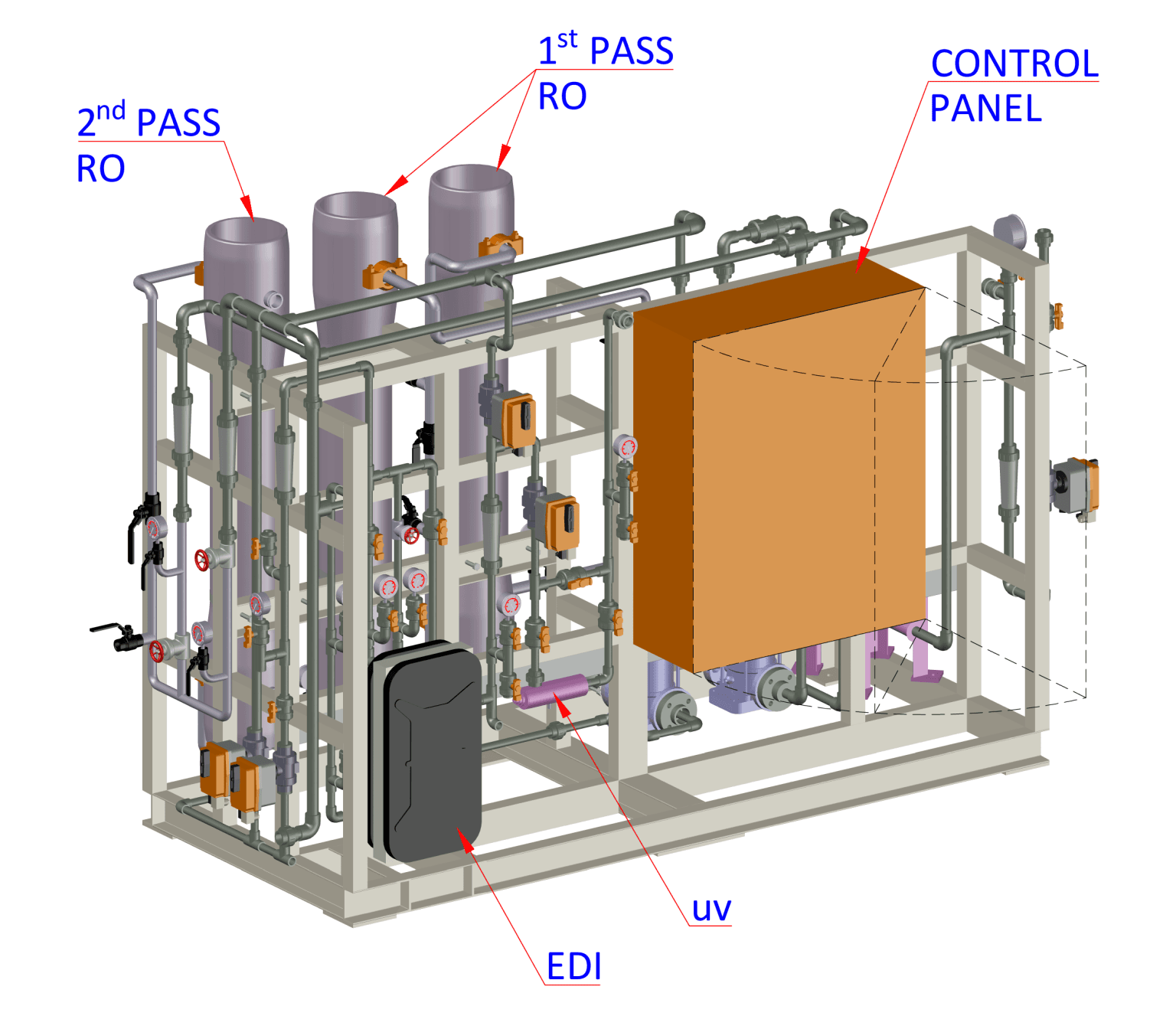
A Sterile Water Production Plant produces water for various uses including Central Sterile Supply Department (CSSD), Pathology, Endoscopy, Dialysis & others. It has a 2-Pass RO & EDI to produce mineral free water & UV disinfection to keep the water sterile. The SWPP coupled with SWDL systems takes care of your water needs.
Sterile Water Production Plant (SWPP)
For AS 5369 Table 7.2, 7.3, 7.4 or AAMI/ISO 13959 Compliant Water