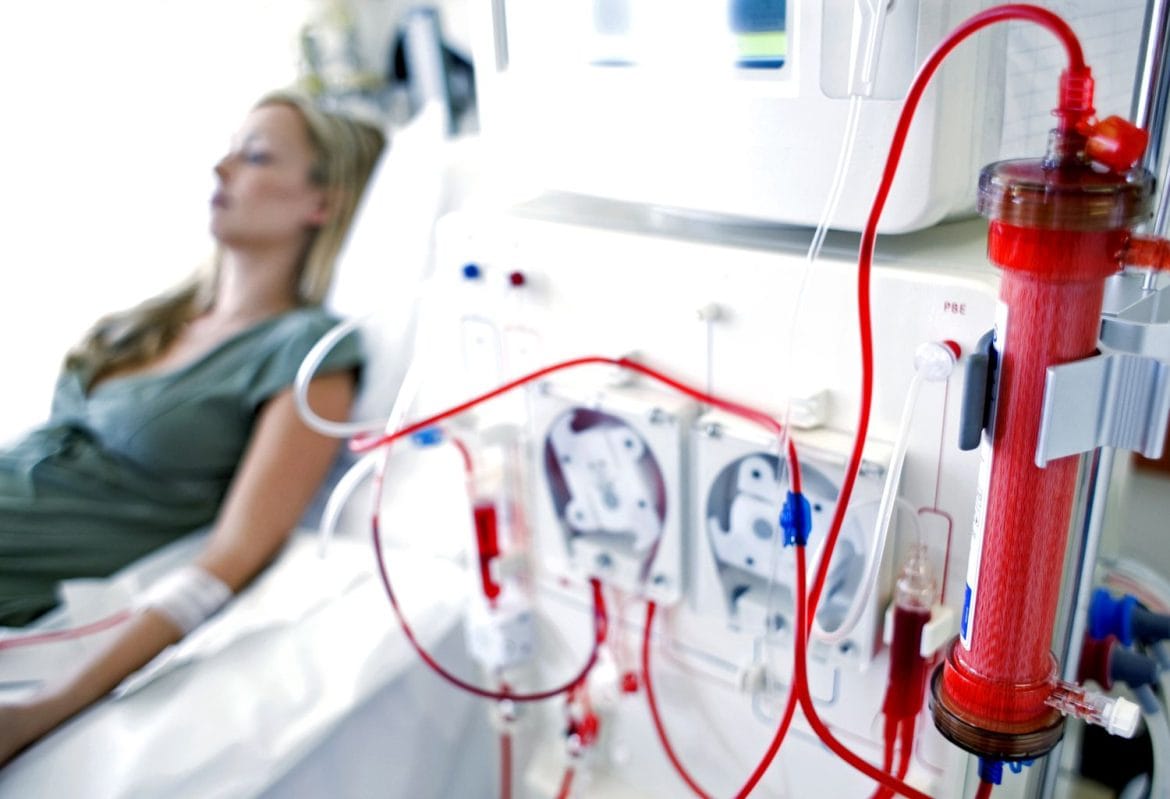
Photo from Dialysis Patient Citizens Education Center
When a patient undergoes hemodialysis, the water that underpins this life-saving therapy isn’t just “clean” — it must be ultra-pure, because every liter counts and the margin for error is extremely small. Unlike drinking water, which passes through the gut and other body systems that filter out many contaminants, dialysis water can come into near-direct contact with the patient’s bloodstream via the semi-permeable dialysis membrane.
Why Trace Contaminants Matter in Dialysis
Volume and direct exposure
In a typical hemodialysis session, a patient may be exposed to 120-200 liters of water (via the dialysate) in a single treatment.
Lack of normal renal clearance
Dialysis patients generally have little to no kidney function. That means many contaminants that a healthy person might eliminate are no longer removed efficiently.
Semi-permeable membrane & diffusion risk
The dialysis membrane is designed to allow diffusion and ultrafiltration of waste products from blood into dialysate. But the flip side: small molecular-weight or ionic contaminants in the water/dialysate may diffuse back into bloodstream, or at least have a route of exposure.
Clinical consequences are real
Various chemical and microbiological contaminants have been liked with acute and chronic adverse events: hemolysis, bone disease, encephalopathy, inflammation, pyrogenic reactions.
For instance:
Elevated aluminum in dialysis water → bone disease, encephalopathy, anemia
Chloramine/chlorine breakdown products → hemolytic anemia
Bacterial endotoxins in dialysate → inflammatory responses, pyrogenic reactions.Because of this, dialysis water treatment must adhere to far stricter standards than typical drinking water quality.
Is Your Dialysis Center’s Water System Safe, Compliant, and Reliable?
Hemodialysis requires ultrapure water to ensure patient safety and equipment longevity. Any compromise in water quality can lead to serious health risks and costly operational downtime.
Whether you’re starting a new fcility or upgrading an existing one, we provide specialized water treatment solutions tailored to dialysis centers. Our systems are built to meet DOH standards and ensure uninterrupted, safe treatment—even during supply disruptions.
Ready to take the next step? Fill out our contact form and let our experts guide you toward a safer,more reliable water solution for your dialysis operations.