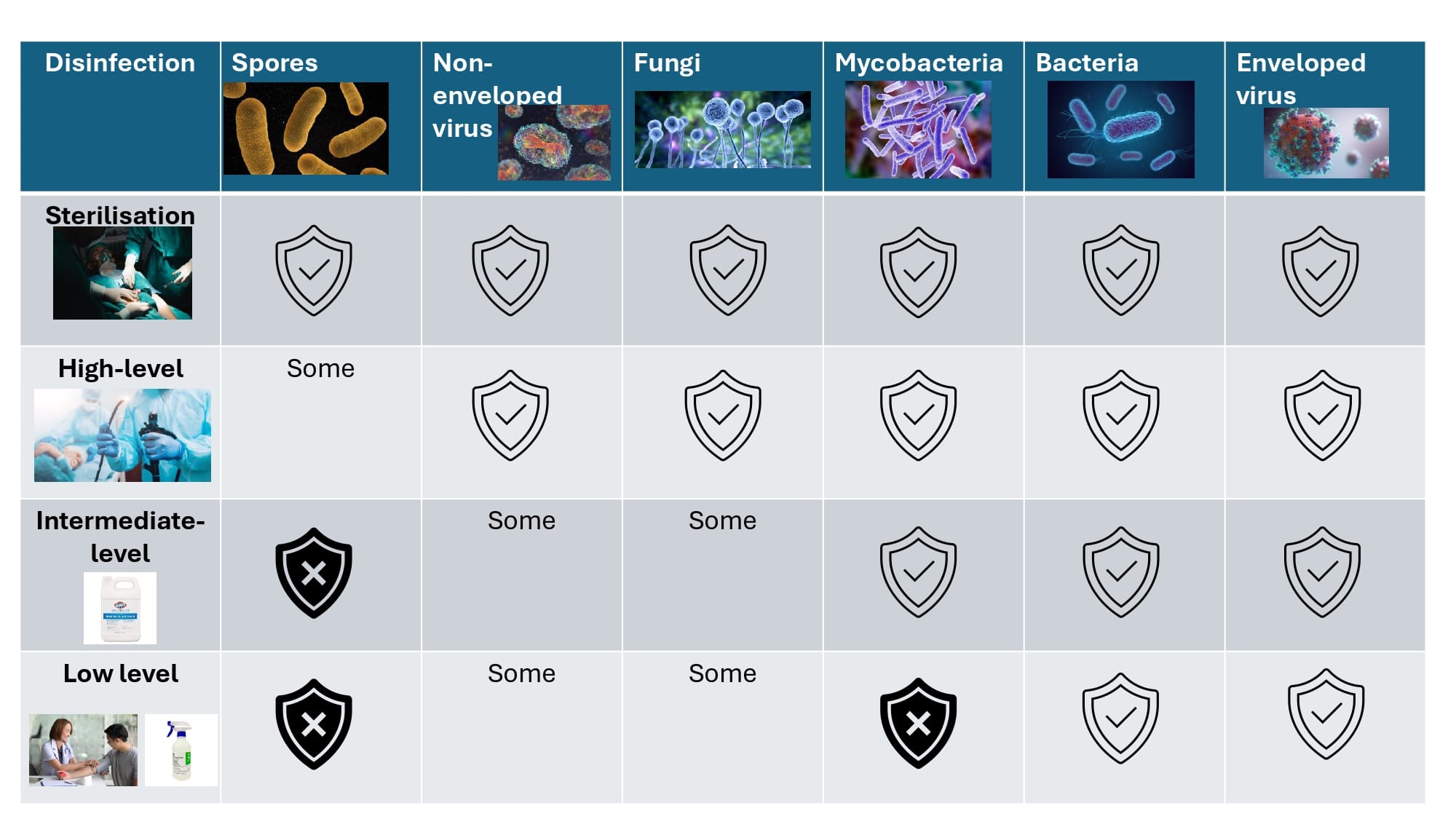
Earle H. Spaulding proposed for an internationally recognised disinfection framework was required since all reusable medical devices (RMDs) cannot be sterilised.
The Spaulding Classification stratifies the risk of infection transmission based on the patient tissue the device will contact during use.
Spaulding Classification | Medical Device Contacts | Risk of Infection Transmission | Disinfection Level |
Critical | Sterile tissue or the bloodstream | High | Sterilisation |
Semi-critical | Mucous membranes or non-intact skin | Medium | High Level Disinfection (HLD) |
Non-critical | Intact skin only | Low | Intermediate level (ILD) or Low level disinfection (LLD) |
Understanding & Recognising Disinfection Levels and Sterilisation
Correctly applying Spaulding Classification to medical devices is a key part of keeping patients safe from healthcare-associated infections (HAIs).
Sterilisation
- Sterilisation destroys all microorganisms.
- Critical devices must be sterile when used.
- Heat sterilization, including steam or hot air.
(Refer to manufacturer’s recommendations, steam sterilization processing time from 3-30 minutes)
High Level Disinfection (HLD)
- HLD destroys all microorganisms except for high numbers of bacterial spores.
- A high-level disinfectant is therefore bactericidal, virucidal (both lipid and non-lipid viruses), fungicidal and mycobactericidal.
- Semi-critical ultrasound probes must undergo HLD and be used with a sheath.
- Critical ultrasound probes that cannot be sterilised can also undergo HLD.
- They must also be used with a sterile sheath.

Ready to Transform Your Water?
If you’re looking to elevate your facility’s water standards, you’ve come to the right place.
To protect the sensitive medical devices that require frequent cleaning and sterilization, hospitals must install water treatment systems designed to treat municipal water supplied to the sterile processing systems (e.g., steam sterilizers, ultrasonic cleaners, washers, sinks etc.)
Let us help you achieve industry compliance while enhancing operational efficiency.
Simply fill out our contact form, and one of our specialists will guide you through the process of transforming your water quality.
